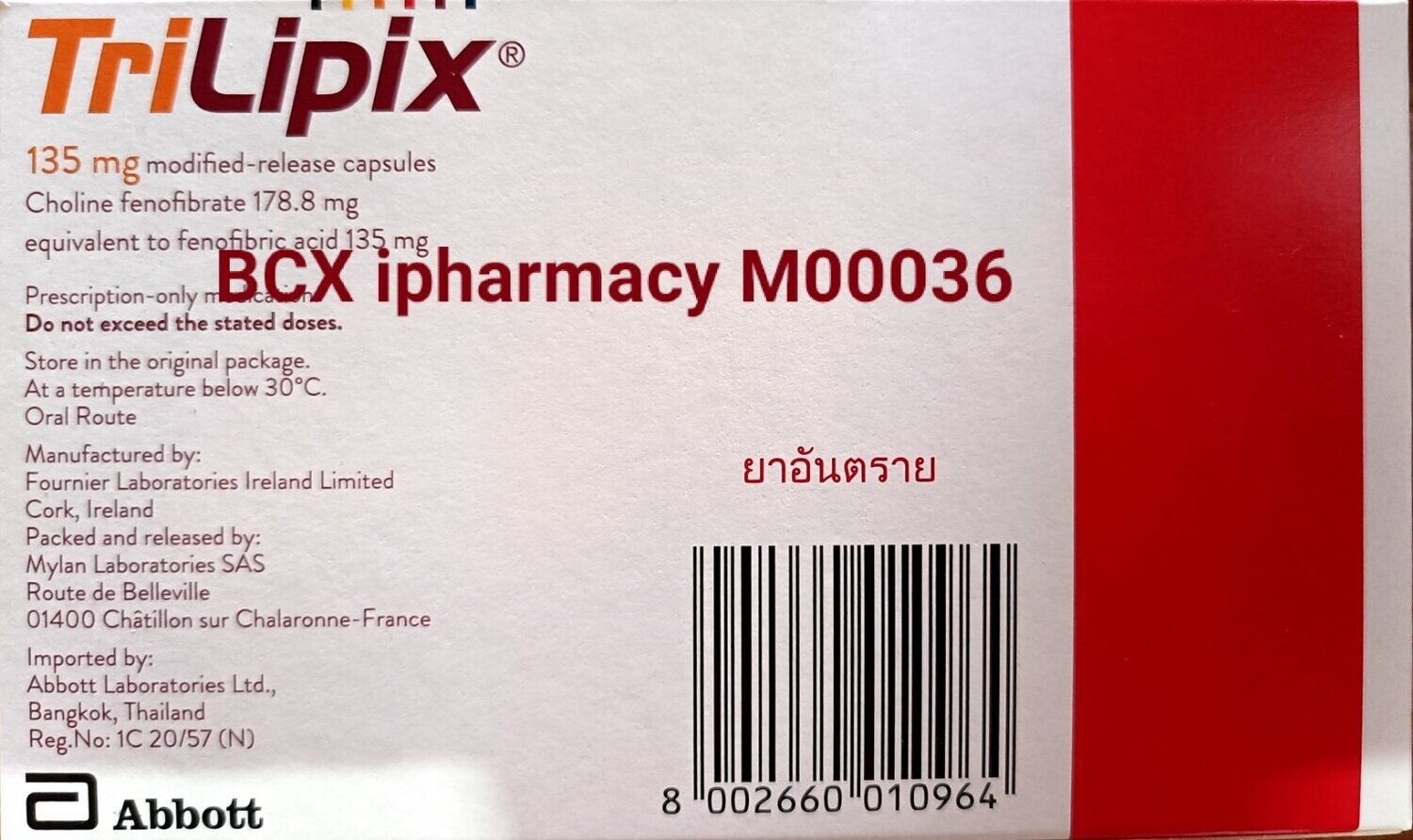
Triplipix 135mg
Ks59,000.00
Pre order only-Selling by
In stock
Product Details
Brand: Manufactured by Fournier Lab, France and Imported by Abbot, Thailand
အဆီကျဆေးဖြစ်ပြီး ဆရာဝန် ညွှန်ကြားချက်ဖြင့်သောက်ရန် လိုအပ်ပါသည်။
Contents
Choline fenofibrate.
Description
Each capsule contains the following excipients: Capsule Content: Hypromellose, povidone, hydroxypropyl cellulose, colloidal silicon dioxide, sodium stearyl fumarate, methacrylic acid copolymer, talc, triethyl citrate (E1505). Capsule Shell 45 mg: Yellow iron oxide (E172), titanium dioxide (E172), black iron oxide (E172), red iron oxide (E172), gelatin. Capsule Shell 135 mg: Yellow iron oxide (E172), titanium dioxide (E171), FD&C Blue #2, gelatin.
Action
Pharmacotherapeutic Group: Serum lipid-reducing agents, cholesterol and triglycerides reducers, fibrates. ATC Code: C10AB.
Pharmacology: Pharmacodynamics: The active moiety of Trilipix is fenofibric acid. The pharmacological effects of fenofibric acid in both animals and humans have been extensively studied through oral administration of fenofibrate.
The lipid-modifying effects of fenofibric acid seen in clinical practice have been explained in vivo in transgenic mice and in vitro in human hepatocyte cultures by the activation of peroxisome proliferator activated receptorα (PPARα). Through this mechanism, fenofibric acid increases lipolysis and elimination of triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of Apo CIII (an inhibitor of lipoprotein lipase activity).
The resulting decrease in tryglycerides produces an alteration in the size and composition of LDL from small, dense particles (which are thought to be atherogenic due to their susceptibility to oxidation), to large buoyant particles. These larger particles have a greater affinity for cholesterol receptors and are catabolized rapidly. Activation of PPARα also induces an increase in the synthesis of HDL-C and Apo AI and AII.
Elevated levels of Total-C, LDL-C and Apo B and decreased levels of HDL-C and its transport complex, Apo AI and Apo AII are risk factors for human atherosclerosis. Epidemiologic studies have established that cardiovascular morbidity and mortality vary directly with the levels of Total-C, LDL-C and triglycerides and inversely with the level of HDL-C. The independent effect of raising HDL-C or lowering triglycerides on the risk of cardiovascular morbidity and mortality has not been determined.
Pharmacokinetics: Trilipix contains fenofibric acid, which is the only circulating pharmacologically active moiety in plasma after oral administration of Trilipix. Fenofibric acid is also the circulating pharmacologically active moiety in plasma after oral administration of fenofibrate, the ester of fenofibric acid.
Plasma concentrations of fenofibric acid after administration of 1 Trilipix 135 mg delayed-release capsule are equivalent to those after one 200 mg capsule of micronized fenofibrate administered under fed conditions.
Absorption: Fenofibric acid is well-absorbed throughout the gastrointestinal tract. The absolute bioavailabilty of fenofibric acid is approximately 81%.
Peak plasma levels of fenofibric acid occur within 4-5 hrs after single dose administration of Trilipix capsule under fasting conditions.
Fenofibric acid exposure in plasma, as measured by maximum peak concentrations (Cmax) and area under the curve (AUC), is not significantly different when a single Trilipix 135 mg dose is administered under fasting or non-fasting conditions.
Distribution: Upon multiple dosing of Trilipix, fenofibric acid levels reach steady state within 8 days. Plasma concentrations of fenofibric acid at steady state are approximately slightly more than double those following a single dose. Serum protein-binding is approximately 99% in normal and dyslipidemic subjects.
Metabolism: Fenofibric acid primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine.
In vivo metabolism data after fenofibrate administration indicate that fenofibric acid does not undergo oxidative metabolism (eg, cytochrome P450) to a significant extent.
Excretion: After absorption, Trilipix is primarily excreted in the urine in the form of fenofibric acid and fenofibric acid glucuronide.
Fenofibric acid is eliminated with a half-life (t½) of approximately 20 hrs, allowing once-daily administration of Trilipix.
Special Populations: Geriatrics: In 5 elderly volunteers 77-87 years, the oral clearance of fenofibric acid following a single oral dose of fenofibrate was 1.2 L/hr, which compared to 1.1 L/hr in young adults. This indicates that an equivalent dose of Trilipix can be used in elderly subjects with normal renal function, without increasing accumulation of the drug or metabolites.
Renal Impairment: The pharmacokinetics of fenofibric acid was examined in patients with mild, moderate and severe renal impairment. Patients with severe renal impairment [creatinine clearance (CrCl) <30 mL/min] showed a 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared to that of healthy subjects. Patients with mild to moderate renal impairment (CrCl 30-80 mL/min) had similar exposure but an increase in the t½ for fenofibric acid compared to that of healthy subjects. Based on these findings, the use of Trilipix should be avoided in patients who have severe renal impairment and dose reduction in patients having mild to moderate renal impairment.
Toxicology: Fenofibric Acid: Because fenofibrate is rapidly converted to its active metabolite, fenofibric acid, either during or immediately following absorption both in animals and humans, studies conducted with fenofibrate are relevant for the assessment of the toxicity profile of fenofibric acid. The systemic toxicity of fenofibrate and fenofibric acid in animal studies is comparable.
Fenofibrate: Chronic toxicity studies have yielded no relevant information about specific toxicity of fenofibrate. Studies on mutagenicity of fenofibrate have been negative.
In rats and mice, liver tumours have been found at high doses, which are attributable to peroxisome proliferation. These changes are specific to small rodents and have not been observed in other animal species. This is of no relevance to therapeutic use in man.
Studies in mice, rats and rabbits did not reveal any teratogenic effect. Embryonic effects were observed at doses in the range of maternal toxicity. Prolongation of the gestation period and difficulties during delivery were observed at high doses. No sign of any effect on fertility has been detected.
Pharmacology: Pharmacodynamics: The active moiety of Trilipix is fenofibric acid. The pharmacological effects of fenofibric acid in both animals and humans have been extensively studied through oral administration of fenofibrate.
The lipid-modifying effects of fenofibric acid seen in clinical practice have been explained in vivo in transgenic mice and in vitro in human hepatocyte cultures by the activation of peroxisome proliferator activated receptorα (PPARα). Through this mechanism, fenofibric acid increases lipolysis and elimination of triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of Apo CIII (an inhibitor of lipoprotein lipase activity).
The resulting decrease in tryglycerides produces an alteration in the size and composition of LDL from small, dense particles (which are thought to be atherogenic due to their susceptibility to oxidation), to large buoyant particles. These larger particles have a greater affinity for cholesterol receptors and are catabolized rapidly. Activation of PPARα also induces an increase in the synthesis of HDL-C and Apo AI and AII.
Elevated levels of Total-C, LDL-C and Apo B and decreased levels of HDL-C and its transport complex, Apo AI and Apo AII are risk factors for human atherosclerosis. Epidemiologic studies have established that cardiovascular morbidity and mortality vary directly with the levels of Total-C, LDL-C and triglycerides and inversely with the level of HDL-C. The independent effect of raising HDL-C or lowering triglycerides on the risk of cardiovascular morbidity and mortality has not been determined.
Pharmacokinetics: Trilipix contains fenofibric acid, which is the only circulating pharmacologically active moiety in plasma after oral administration of Trilipix. Fenofibric acid is also the circulating pharmacologically active moiety in plasma after oral administration of fenofibrate, the ester of fenofibric acid.
Plasma concentrations of fenofibric acid after administration of 1 Trilipix 135 mg delayed-release capsule are equivalent to those after one 200 mg capsule of micronized fenofibrate administered under fed conditions.
Absorption: Fenofibric acid is well-absorbed throughout the gastrointestinal tract. The absolute bioavailabilty of fenofibric acid is approximately 81%.
Peak plasma levels of fenofibric acid occur within 4-5 hrs after single dose administration of Trilipix capsule under fasting conditions.
Fenofibric acid exposure in plasma, as measured by maximum peak concentrations (Cmax) and area under the curve (AUC), is not significantly different when a single Trilipix 135 mg dose is administered under fasting or non-fasting conditions.
Distribution: Upon multiple dosing of Trilipix, fenofibric acid levels reach steady state within 8 days. Plasma concentrations of fenofibric acid at steady state are approximately slightly more than double those following a single dose. Serum protein-binding is approximately 99% in normal and dyslipidemic subjects.
Metabolism: Fenofibric acid primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine.
In vivo metabolism data after fenofibrate administration indicate that fenofibric acid does not undergo oxidative metabolism (eg, cytochrome P450) to a significant extent.
Excretion: After absorption, Trilipix is primarily excreted in the urine in the form of fenofibric acid and fenofibric acid glucuronide.
Fenofibric acid is eliminated with a half-life (t½) of approximately 20 hrs, allowing once-daily administration of Trilipix.
Special Populations: Geriatrics: In 5 elderly volunteers 77-87 years, the oral clearance of fenofibric acid following a single oral dose of fenofibrate was 1.2 L/hr, which compared to 1.1 L/hr in young adults. This indicates that an equivalent dose of Trilipix can be used in elderly subjects with normal renal function, without increasing accumulation of the drug or metabolites.
Renal Impairment: The pharmacokinetics of fenofibric acid was examined in patients with mild, moderate and severe renal impairment. Patients with severe renal impairment [creatinine clearance (CrCl) <30 mL/min] showed a 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared to that of healthy subjects. Patients with mild to moderate renal impairment (CrCl 30-80 mL/min) had similar exposure but an increase in the t½ for fenofibric acid compared to that of healthy subjects. Based on these findings, the use of Trilipix should be avoided in patients who have severe renal impairment and dose reduction in patients having mild to moderate renal impairment.
Toxicology: Fenofibric Acid: Because fenofibrate is rapidly converted to its active metabolite, fenofibric acid, either during or immediately following absorption both in animals and humans, studies conducted with fenofibrate are relevant for the assessment of the toxicity profile of fenofibric acid. The systemic toxicity of fenofibrate and fenofibric acid in animal studies is comparable.
Fenofibrate: Chronic toxicity studies have yielded no relevant information about specific toxicity of fenofibrate. Studies on mutagenicity of fenofibrate have been negative.
In rats and mice, liver tumours have been found at high doses, which are attributable to peroxisome proliferation. These changes are specific to small rodents and have not been observed in other animal species. This is of no relevance to therapeutic use in man.
Studies in mice, rats and rabbits did not reveal any teratogenic effect. Embryonic effects were observed at doses in the range of maternal toxicity. Prolongation of the gestation period and difficulties during delivery were observed at high doses. No sign of any effect on fertility has been detected.
Indications/Uses
As an adjunct to diet: In combination with a statin to reduce triglycerides and increase HDL-C in patients with mixed dyslipidemia and CHD or a CHD risk equivalent (other clinical forms of atherosclerotic disease: Peripheral arterial disease, abdominal aortic aneurysm and symptomatic carotid artery disease; diabetes; multiple risk factors that confer a 10-year risk for CHD >20%) who are on optimal statin therapy to achieve their LDL-C.
To reduce trigycerides in patients with severe hypertriglyceridemia.
To reduce elevated LDL-C, total cholesterol, triglycerides and Apo B and increase HDL-C in patients with primary hyperlipidemia or mixed dyslipidemia.
No incremental benefit of Trilipix on cardiovascular morbidity and mortality over and above that demonstrated for statin monotherapy has been established.
Fenofibrate at a dose equivalent to Trilipix 135 mg was not shown to reduce coronary heart disease morbidity and mortality in a large, randomized controlled trial of patients with type 2 diabetes mellitus.
To reduce trigycerides in patients with severe hypertriglyceridemia.
To reduce elevated LDL-C, total cholesterol, triglycerides and Apo B and increase HDL-C in patients with primary hyperlipidemia or mixed dyslipidemia.
No incremental benefit of Trilipix on cardiovascular morbidity and mortality over and above that demonstrated for statin monotherapy has been established.
Fenofibrate at a dose equivalent to Trilipix 135 mg was not shown to reduce coronary heart disease morbidity and mortality in a large, randomized controlled trial of patients with type 2 diabetes mellitus.
Dosage/Direction for Use
Patients should be placed on an appropriate lipid-lowering diet before receiving Trilipix as monotherapy or co-administered with a statin and should continue this diet during treatment.
Trilipix modified-release capsules can be taken without regard to meals. Serum lipids should be monitored periodically.
Maximum Dose: 135 mg once daily.
Adults: Co-Administration with Statins for the Treatment of Mixed Dyslipidemia: Trilipix 135 mg may be co-administered with an HMG-CoA reductase inhibitor (statin) in patients with mixed dyslipidemia. For convenience, the daily dose of Trilipix may be taken at the same time as a statin, according to the dosing recommendations for each medication. Co-administration with the maximum dose of a statin has not been evaluated in clinical studies and should be avoided unless the benefits are expected to outweigh the risks.
Severe Hypertriglyceridemia: Initially 45-135 mg once daily. Dosage should be individualized according to patient response and should be adjusted, if necessary, following repeat lipid determinations at 4-8 week intervals. Maximum Dose: 135 mg once daily.
Primary Hyperlipidemia or Mixed Dyslipidemia: 135 mg once daily.
Elderly: Dose selection for the elderly should be made on the basis of renal function.
Renal Impairment: Treatment with Trilipix should be initiated at a dose of 45 mg once daily in patients with mild to moderate renal impairment [creatinine clearance (CrCl) 30-80 mL/min] and should only be increased after evaluation of the effects on renal function and lipid levels at this dose. The use of Trilipix should be avoided in patients with severely impaired renal function.
Hepatic Impairment: Patients with hepatic disease have not been studied.
Trilipix modified-release capsules can be taken without regard to meals. Serum lipids should be monitored periodically.
Maximum Dose: 135 mg once daily.
Adults: Co-Administration with Statins for the Treatment of Mixed Dyslipidemia: Trilipix 135 mg may be co-administered with an HMG-CoA reductase inhibitor (statin) in patients with mixed dyslipidemia. For convenience, the daily dose of Trilipix may be taken at the same time as a statin, according to the dosing recommendations for each medication. Co-administration with the maximum dose of a statin has not been evaluated in clinical studies and should be avoided unless the benefits are expected to outweigh the risks.
Severe Hypertriglyceridemia: Initially 45-135 mg once daily. Dosage should be individualized according to patient response and should be adjusted, if necessary, following repeat lipid determinations at 4-8 week intervals. Maximum Dose: 135 mg once daily.
Primary Hyperlipidemia or Mixed Dyslipidemia: 135 mg once daily.
Elderly: Dose selection for the elderly should be made on the basis of renal function.
Renal Impairment: Treatment with Trilipix should be initiated at a dose of 45 mg once daily in patients with mild to moderate renal impairment [creatinine clearance (CrCl) 30-80 mL/min] and should only be increased after evaluation of the effects on renal function and lipid levels at this dose. The use of Trilipix should be avoided in patients with severely impaired renal function.
Hepatic Impairment: Patients with hepatic disease have not been studied.
Overdosage
There is no specific treatment for overdose with Trilipix. General supportive care of the patient is indicated, including monitoring of vital signs and observation of clinical status, should an overdose occur. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. Because Trilipix is highly bound to plasma proteins, hemodialysis should not be considered.
Contraindications
Hypersensitivity to fenofibric acid, choline fenofibrate, fenofibrate or to any of the excipients of Trilipix. Known photoallergy or phototoxic reaction during treatment with fibrates or ketoprofen; severe renal insufficiency (CrCl <30 mL/min); hepatic insufficiency (including biliary cirrhosis and unexplained persistent liver function abnormality); gallbladder disease; chronic or acute pancreatitis with the exception of acute pancreatitis due to severe hypertriglyceridemia.
Special Precautions
Skeletal Muscles: Fibrate and statin monotherapy increase the risk of myositis or myopathy, and have been associated with rhabdomyolysis. Data from observational studies suggest that the risk for rhabdomyolysis is increased when fibrates are co-administered with a statin. Refer to the respective statin labeling for important drug-drug interactions that increase statin levels and could increase this risk. The risk for serious muscle toxicity appears to be increased in elderly patients and in patients with diabetes, renal failure or hypothyroidism. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness and/or marked elevations of CPK levels. Patients should promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. CPK levels should be assessed in patients reporting these symptoms, and Trilipix and statin therapy should be discontinued if markedly elevated CPK levels (level exceeding 5 times the upper limit of the normal range) occur or myopathy or myositis is diagnosed.
Renal Function: Reversible elevations in serum creatinine have been reported in patients receiving Trilipix as monotherapy or co-administered with statins as well as patients receiving fenofibrate. Elevations in serum creatinine were generally stable over time with no evidence for continued increases in serum creatinine with long-term therapy and tended to return to baseline following discontinuation of treatment. The clinical significance of these observations is unknown. Monitoring renal function in patients with renal impairment taking Trilipix is suggested. Renal monitoring should be considered for patients at risk for renal insufficiency, eg, the elderly and those with diabetes. Treatment should be interrupted in case of an increase in creatinine levels of >50% upper limit of normal. It is recommended that creatinine is measured during the first 3 months after initiation of treatment and thereafter periodically.
Liver Function: Trilipix at a dose of 135 mg once daily administered as monotherapy or co-administered with low to moderate doses of statins has been associated with increases in serum transaminases [aspartate aminotransferase (AST) serum glutamic-oxaloacetic transaminase (SGOT) or alanine aminotransferase (ALT) serum glutamic-pyruvic transaminase (SGPT)]. Hepatocellular, chronic active and cholestatic hepatitis observed with fenofibrate therapy have been reported after exposures of weeks to several years. In extremely rare cases, cirrhosis has been reported in association with chronic active hepatitis.
Regular monitoring of liver function including serum ALT (SGPT) and AST (SGPT) should be performed periodically for the duration of therapy with Trilipix, and therapy discontinued if enzyme levels persist >3 times the upper limit of normal.
Pancreatitis: Pancreatitis has been reported in patients taking drugs of the fibrate class, including Trilipix. This occurrence may represent a failure of efficacy in patients with severe hypertriglyceridemia, a direct drug effect, or a secondary phenomenon mediated through biliary tract stone or sludge formation with obstruction of the common bile duct.
Use in Pregnancy: There are no adequate data from the use of Trilipix in pregnant women. The potential risk for humans is unknown. Trilipix should not be used during pregnancy unless clearly necessary.
Effects on the Ability to Drive or Operate Machinery: Trilipix has no or negligible influence on the ability to drive and use machines.
Use in Lactation: It is unknown whether fenofibric acid is excreted in human breast milk. The excretion of fenofibric acid in milk has not been studied in animals. A decision on whether to continue/discontinue breastfeeding or to continue/discontinue therapy with Trilipix should be made taking into account the benefit of breastfeeding to the child and the benefit of Trilipix therapy to the woman.
Use in Children: Trilipix is not recommended for use in children <18 years due to lack of data on safety and efficacy.
Renal Function: Reversible elevations in serum creatinine have been reported in patients receiving Trilipix as monotherapy or co-administered with statins as well as patients receiving fenofibrate. Elevations in serum creatinine were generally stable over time with no evidence for continued increases in serum creatinine with long-term therapy and tended to return to baseline following discontinuation of treatment. The clinical significance of these observations is unknown. Monitoring renal function in patients with renal impairment taking Trilipix is suggested. Renal monitoring should be considered for patients at risk for renal insufficiency, eg, the elderly and those with diabetes. Treatment should be interrupted in case of an increase in creatinine levels of >50% upper limit of normal. It is recommended that creatinine is measured during the first 3 months after initiation of treatment and thereafter periodically.
Liver Function: Trilipix at a dose of 135 mg once daily administered as monotherapy or co-administered with low to moderate doses of statins has been associated with increases in serum transaminases [aspartate aminotransferase (AST) serum glutamic-oxaloacetic transaminase (SGOT) or alanine aminotransferase (ALT) serum glutamic-pyruvic transaminase (SGPT)]. Hepatocellular, chronic active and cholestatic hepatitis observed with fenofibrate therapy have been reported after exposures of weeks to several years. In extremely rare cases, cirrhosis has been reported in association with chronic active hepatitis.
Regular monitoring of liver function including serum ALT (SGPT) and AST (SGPT) should be performed periodically for the duration of therapy with Trilipix, and therapy discontinued if enzyme levels persist >3 times the upper limit of normal.
Pancreatitis: Pancreatitis has been reported in patients taking drugs of the fibrate class, including Trilipix. This occurrence may represent a failure of efficacy in patients with severe hypertriglyceridemia, a direct drug effect, or a secondary phenomenon mediated through biliary tract stone or sludge formation with obstruction of the common bile duct.
Use in Pregnancy: There are no adequate data from the use of Trilipix in pregnant women. The potential risk for humans is unknown. Trilipix should not be used during pregnancy unless clearly necessary.
Effects on the Ability to Drive or Operate Machinery: Trilipix has no or negligible influence on the ability to drive and use machines.
Use in Lactation: It is unknown whether fenofibric acid is excreted in human breast milk. The excretion of fenofibric acid in milk has not been studied in animals. A decision on whether to continue/discontinue breastfeeding or to continue/discontinue therapy with Trilipix should be made taking into account the benefit of breastfeeding to the child and the benefit of Trilipix therapy to the woman.
Use in Children: Trilipix is not recommended for use in children <18 years due to lack of data on safety and efficacy.
Use In Pregnancy & Lactation
Use in Pregnancy: There are no adequate data from the use of Trilipix in pregnant women. The potential risk for humans is unknown. Trilipix should not be used during pregnancy unless clearly necessary.
Use in Lactation: It is unknown whether fenofibric acid is excreted in human breast milk. The excretion of fenofibric acid in milk has not been studied in animals. A decision on whether to continue/discontinue breastfeeding or to continue/discontinue therapy with Trilipix should be made taking into account the benefit of breastfeeding to the child and the benefit of Trilipix therapy to the woman.
Use in Lactation: It is unknown whether fenofibric acid is excreted in human breast milk. The excretion of fenofibric acid in milk has not been studied in animals. A decision on whether to continue/discontinue breastfeeding or to continue/discontinue therapy with Trilipix should be made taking into account the benefit of breastfeeding to the child and the benefit of Trilipix therapy to the woman.
Adverse Reactions
Clinical studies experience with Trilipix (fenofibric acid).
Monotherapy: Treatment-emergent adverse events reported in ≥3% of patients treated with Trilipix during the randomized controlled trials are listed in table.
Co-administration Therapy with Statins (Double-Blind Controlled Trials): Treatment-emergent adverse events reported in ≥3% of patients treated with Trilipix co-administered with statins during the randomized controlled trials are listed in table.
 Click on icon to see table/diagram/image
Click on icon to see table/diagram/image
Co-Administration Therapy with Statins (Long-Term Exposure for up to 64 Weeks): Patients successfully completing any 1 of the 3 double-blind, controlled studies were eligible to participate in a 52-week long-term extension study where they received Trilipix co-administered with the moderate dose statin. A total of 2201 patients received at least 1 dose of Trilipix co-administered with a statin in the double-blind controlled study or the long-term extension study for up to a total of 64 weeks of treatment. Additional treatment-emergent adverse events (not listed in table as previously mentioned) reported in ≥3% of patients receiving Trilipix co-administered with a statin in either the double-blind controlled studies or the long-term extension study are provided as follows: Infections and Infestations: Bronchitis, influenza and urinary tract infection.
Investigations: Increased AST, increased blood CPK and increased hepatic enzyme.
Musculoskeletal and Connective Tissue Disorders: Musculoskeletal pain.
Psychiatric Disorders: Insomnia.
Respiratory, Thoracic and Mediastinal Disorders: Cough and pharyngolaryngeal pain.
Vascular Disorders: Hypertension.
Fenofibrate: Fenofibric acid is the active metabolite of fenofibrate. The following undesirable effects have been observed during placebo-controlled clinical trials using fenofibrate (n=2344) with frequencies: Common (>1/100 to <1/10); uncommon (>1/1000 to <1/100); rare (>1/10,000 to <1/1000); very rare (<1/10,000 including isolated reports).
Blood Lymphatic System Disorders: Rare: Decrease haemoglobin and white blood cell count.
Immune System Disorders: Rare: Hypersensitivity.
Nervous System Disorders: Uncommon: Headache.
Vascular Disorders: Uncommon: Thromboembolism (pulmonary embolism, deep vein thrombosis)*.
Gastrointestinal Disorders: Common: Gastrointestinal signs and symptoms (abdominal pain, nausea, vomiting, diarrhoea, flatulence). Uncommon: Pancreatitis*.
Hepatobiliary Disorders: Common: Increased transaminases. Uncommon: Cholelithiasis. Rare: Hepatitis.
Skin and Subcutaneous Tissue Disorders: Uncommon: Cutaneous hypersensitivity (eg, rashes, pruritus, urticaria). Rare: Alopecia, photosensitivity reactions.
Musculoskeletal, Connective Tissue and Bone Disorders: Uncommon: Muscle disorder (eg, myalgia, myositis, muscular spasms and weakness).
Reproductive System and Breast Disorders: Uncommon: Sexual dysfunction.
Investigations: Uncommon: Increased blood creatinine. Rare: Increased blood urea.
*In the FIELD-study, a randomized placebo-controlled trial performed in 9795 patients with type 2 diabetes mellitus, a statistically significant increase in pancreatitis cases was observed in patients receiving fenofibrate versus patients receiving placebo (0.8% vs 0.5%; p=0.031). In the same study, a statistically significant increase was reported in the incidence of pulmonary embolism (0.7% in the placebo group vs 1.1% in the fenofibrate group; p=0.022) and a statistically non-significant increase in deep vein thromboses [placebo: 1% (48/4900 patients) vs fenofibrate 1.4% (67/4895 patients); p=0.074].
In addition to those events reported during clinical trials, the following side effects have been reported spontaneously during post-marketing use of fenofibrate. A precise frequency cannot be estimated from the available data and is therefore classified as “not known”.
Respiratory, Thoracic and Mediastinal Disorders: Interstitial lung disease.
Musculoskeletal, Connective Tissue and Bone Disorders: Rhabdomyolysis.
Monotherapy: Treatment-emergent adverse events reported in ≥3% of patients treated with Trilipix during the randomized controlled trials are listed in table.
Co-administration Therapy with Statins (Double-Blind Controlled Trials): Treatment-emergent adverse events reported in ≥3% of patients treated with Trilipix co-administered with statins during the randomized controlled trials are listed in table.
 Click on icon to see table/diagram/image
Click on icon to see table/diagram/image
Co-Administration Therapy with Statins (Long-Term Exposure for up to 64 Weeks): Patients successfully completing any 1 of the 3 double-blind, controlled studies were eligible to participate in a 52-week long-term extension study where they received Trilipix co-administered with the moderate dose statin. A total of 2201 patients received at least 1 dose of Trilipix co-administered with a statin in the double-blind controlled study or the long-term extension study for up to a total of 64 weeks of treatment. Additional treatment-emergent adverse events (not listed in table as previously mentioned) reported in ≥3% of patients receiving Trilipix co-administered with a statin in either the double-blind controlled studies or the long-term extension study are provided as follows: Infections and Infestations: Bronchitis, influenza and urinary tract infection.
Investigations: Increased AST, increased blood CPK and increased hepatic enzyme.
Musculoskeletal and Connective Tissue Disorders: Musculoskeletal pain.
Psychiatric Disorders: Insomnia.
Respiratory, Thoracic and Mediastinal Disorders: Cough and pharyngolaryngeal pain.
Vascular Disorders: Hypertension.
Fenofibrate: Fenofibric acid is the active metabolite of fenofibrate. The following undesirable effects have been observed during placebo-controlled clinical trials using fenofibrate (n=2344) with frequencies: Common (>1/100 to <1/10); uncommon (>1/1000 to <1/100); rare (>1/10,000 to <1/1000); very rare (<1/10,000 including isolated reports).
Blood Lymphatic System Disorders: Rare: Decrease haemoglobin and white blood cell count.
Immune System Disorders: Rare: Hypersensitivity.
Nervous System Disorders: Uncommon: Headache.
Vascular Disorders: Uncommon: Thromboembolism (pulmonary embolism, deep vein thrombosis)*.
Gastrointestinal Disorders: Common: Gastrointestinal signs and symptoms (abdominal pain, nausea, vomiting, diarrhoea, flatulence). Uncommon: Pancreatitis*.
Hepatobiliary Disorders: Common: Increased transaminases. Uncommon: Cholelithiasis. Rare: Hepatitis.
Skin and Subcutaneous Tissue Disorders: Uncommon: Cutaneous hypersensitivity (eg, rashes, pruritus, urticaria). Rare: Alopecia, photosensitivity reactions.
Musculoskeletal, Connective Tissue and Bone Disorders: Uncommon: Muscle disorder (eg, myalgia, myositis, muscular spasms and weakness).
Reproductive System and Breast Disorders: Uncommon: Sexual dysfunction.
Investigations: Uncommon: Increased blood creatinine. Rare: Increased blood urea.
*In the FIELD-study, a randomized placebo-controlled trial performed in 9795 patients with type 2 diabetes mellitus, a statistically significant increase in pancreatitis cases was observed in patients receiving fenofibrate versus patients receiving placebo (0.8% vs 0.5%; p=0.031). In the same study, a statistically significant increase was reported in the incidence of pulmonary embolism (0.7% in the placebo group vs 1.1% in the fenofibrate group; p=0.022) and a statistically non-significant increase in deep vein thromboses [placebo: 1% (48/4900 patients) vs fenofibrate 1.4% (67/4895 patients); p=0.074].
In addition to those events reported during clinical trials, the following side effects have been reported spontaneously during post-marketing use of fenofibrate. A precise frequency cannot be estimated from the available data and is therefore classified as “not known”.
Respiratory, Thoracic and Mediastinal Disorders: Interstitial lung disease.
Musculoskeletal, Connective Tissue and Bone Disorders: Rhabdomyolysis.
View ADR Monitoring Form
Drug Interactions
Oral Anticoagulants: Caution should be exercised when Trilipix is given in conjunction with oral coumarin anticoagulants. Trilipix may potentiate the anticoagulant effects of these agents resulting in prolongation of the prothrombin time/INR. Frequent monitoring of prothrombin time/INR and dose adjustment of the oral anticoagulant are recommended until the prothrombin time/INR has stabilized in order to prevent bleeding complications.
Cyclosporine: Because cyclosporine can produce nephrotoxicity with decreases in creatinine clearance and rises in serum creatinine, and because renal excretion is the primary elimination route of drugs of the fibrate class including Trilipix, there is a risk that an interaction will lead to decline of renal function. The benefits and risks of using Trilipix with immunosuppressants and other potentially nephrotoxic agents should be carefully considered, and the lowest effective dose employed.
Statins: The risk of serious muscle toxicity may be increased if fenofibrate or fenofibric acid is used concomitantly with HMG-CoA reductase inhibitors. Such combination therapy should be used with caution and patients monitored closely for signs of muscle toxicity (see Precautions). Specific studies in healthy volunteers have demonstrated the absence of clinically relevant pharmacokinetic interaction with lipid lowering agents eg, HMG-CoA reductase inhibitors (atorvastatin, fluvastatin, pravastatin, rosuvastatin and simvastatin) and ezetimibe, however, a pharmacodynamic interaction cannot be excluded. No dosing adjustment is then required for Trilipix or the co-administered drugs.
Oral Hypoglycaemic Agents: In healthy volunteers, no clinically relevant pharmacokinetic interactions have been shown between fenofibrate or fenofibric acid and rosiglitazone, metformin or glimepiride. No dosing adjustment is required for Trilipix or the co-administered drugs.
Gastrointestinal Agents: In healthy volunteers, no clinically relevant pharmacokinetic interactions have been shown between fenofibrate or fenofibric acid and omeprazole.
In vitro studies using human liver microsomes indicate that fenofibric acid is not an inhibitor of cytochrome (CYP) P450 isoforms CYP3A4, CYP2D6, CYP2E1, or CYP1A2. It is a weak inhibitor of CYP2C8, CYP2C19 and CYP2A6, and mild-to-moderate inhibitor of CYP2C9 at therapeutic concentrations.
Cyclosporine: Because cyclosporine can produce nephrotoxicity with decreases in creatinine clearance and rises in serum creatinine, and because renal excretion is the primary elimination route of drugs of the fibrate class including Trilipix, there is a risk that an interaction will lead to decline of renal function. The benefits and risks of using Trilipix with immunosuppressants and other potentially nephrotoxic agents should be carefully considered, and the lowest effective dose employed.
Statins: The risk of serious muscle toxicity may be increased if fenofibrate or fenofibric acid is used concomitantly with HMG-CoA reductase inhibitors. Such combination therapy should be used with caution and patients monitored closely for signs of muscle toxicity (see Precautions). Specific studies in healthy volunteers have demonstrated the absence of clinically relevant pharmacokinetic interaction with lipid lowering agents eg, HMG-CoA reductase inhibitors (atorvastatin, fluvastatin, pravastatin, rosuvastatin and simvastatin) and ezetimibe, however, a pharmacodynamic interaction cannot be excluded. No dosing adjustment is then required for Trilipix or the co-administered drugs.
Oral Hypoglycaemic Agents: In healthy volunteers, no clinically relevant pharmacokinetic interactions have been shown between fenofibrate or fenofibric acid and rosiglitazone, metformin or glimepiride. No dosing adjustment is required for Trilipix or the co-administered drugs.
Gastrointestinal Agents: In healthy volunteers, no clinically relevant pharmacokinetic interactions have been shown between fenofibrate or fenofibric acid and omeprazole.
In vitro studies using human liver microsomes indicate that fenofibric acid is not an inhibitor of cytochrome (CYP) P450 isoforms CYP3A4, CYP2D6, CYP2E1, or CYP1A2. It is a weak inhibitor of CYP2C8, CYP2C19 and CYP2A6, and mild-to-moderate inhibitor of CYP2C9 at therapeutic concentrations.
Storage
Shelf-Life: This product can be stored for up 24 months in blister or 36 months in bottle.
MIMS Class
ATC Classification
C10AB11 - choline fenofibrate ; Belongs to the class of fibrates. Used in the treatment of hyperlipidemia.
Display prices in:MMK